Fertility Treatments
We offer different types of treatment adapted to your situation in order to achieve your dream: to give birth to a baby.

In Vitro Fertilization
The process begins with hormone therapy to stimulate the ovaries to produce multiple eggs. This typically involves daily injections of gonadotropins.
During stimulation, about 3 to 5 blood tests and ultrasounds are performed to monitor the growth and development of the follicles (which contain the eggs).
Once the follicles are mature, a trigger shot of hCG or GnRh agonist (intranasal Synarel (r) or subcunateous decapeptyl (r)) is given to prepare for egg retrieval. About 36 hours later, a minor surgical procedure is performed, usually under anesthesia, to collect the eggs through the vaginal wall from the ovaries using a thin needle.
During the egg retrieval, a sperm sample is collected from the male partner or a donor . The sperm is then processed to isolate the healthiest sperm.
The retrieved eggs are combined with the processed sperm in a laboratory dish. Fertilization can occur naturally or through a technique called intracytoplasmic sperm injection (ICSI), where a single sperm is injected directly into an egg.
The fertilized eggs, now called embryos, are cultured for several days (usually 3 to 5 days) to allow them to develop. Embryologists monitor their growth and quality.
Once the embryos are ready, one is selected for transfer into the woman’s uterus. This is done using a thin catheter and is usually a painless procedure. The other day 5 embryos are frozen
Hormonal support, often in the form of progesterone supplements, is provided to help prepare the uterine lining for implantation.
About 10-14 days after the embryo transfer, a blood test is performed to determine if implantation has occurred and if the woman is pregnant.
If the test is positive, additional monitoring occurs to ensure a healthy pregnancy. If not, the woman may discuss options for future cycles.
Each step is crucial for the success of IVF, and individual experiences may vary.
Intrauterine insemination
Intrauterine insemination (IUI) is a medical procedure used to treat infertility. It involves placing sperm directly into the uterus to increase the chances of fertilization. The process typically unfolds in several stages:
The first step involves a thorough evaluation of both partners’ fertility. This may include blood tests, ultrasound examinations, and sperm analysis to determine the underlying causes of infertility and decide if IUI is a suitable treatment option.
To optimize the chances of conception, the woman may be prescribed medications that stimulate her ovaries to produce multiple eggs. Common drugs used for this purpose include clomifene or gonadotropins. The response to these medications is closely monitored with ultrasounds and blood tests to track the growth of follicles (the sacs containing eggs).
Regular ultrasounds are performed to monitor the growth of the follicles. Once the follicles have reached the right size, an injection of human chorionic gonadotropin (hCG) is often given to trigger ovulation, signaling the eggs to be released.
On the day of insemination, a sperm sample is collected from the male partner (or a sperm donor if necessary). The sperm is then processed in the laboratory to separate healthy, motile sperm from the semen. This ensures that the sperm used for insemination are of the highest quality.
Once ovulation is triggered, and the sperm is prepared, the IUI procedure itself takes place. The sperm is carefully inserted directly into the uterus through a thin catheter. This is done under the guidance of a pelvic ultrasound to ensure precise placement. The procedure is relatively quick and typically painless, though some women may experience mild cramping. The procedure can be performed at the usual doctor’s office.
After the procedure, the woman may be advised to rest for a few minutes before resuming normal activities. Blood tests are usually scheduled about 10-14 days later to check for pregnancy. A positive result is confirmed with a follow-up ultrasound.
IUI is a less invasive option compared to other fertility treatments, and although its success rates can vary depending on factors such as age, the cause of infertility, and the quality of sperm, it offers a hopeful solution for many couples facing challenges with conception.

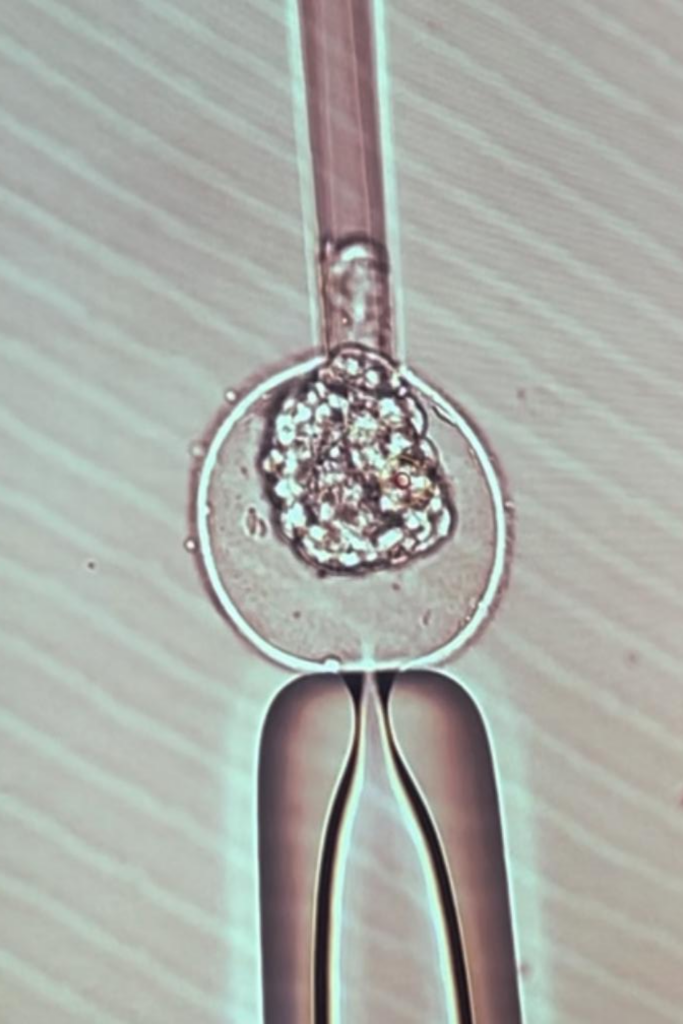
Pgt-a & pgt-m
Preimplantation Genetic Testing for Aneuploidy (PGT-A) and Preimplantation Genetic Testing for Monogenic Disorders (PGT-M) are advanced genetic tests used in conjunction with in vitro fertilization (IVF) to screen embryos for genetic abnormalities before implantation. These procedures aim to increase the chances of a successful pregnancy by identifying embryos with chromosomal or genetic conditions that could lead to miscarriage, genetic disorders, or other complications. Here is an overview of the procedures and indications for both PGT-A and PGT-M:
Egg donation
In vitro fertilization (IVF) with egg donation (often referred to as “egg donation IVF”) is a fertility treatment option where eggs from a donor are used to create embryos that will then be implanted into the recipient’s uterus. This procedure is typically recommended for women who are unable to use their own eggs due to conditions such as premature ovarian failure, poor egg quality, or advanced age. The IVF process with egg donation follows several key stages:
The process begins with an initial consultation with a fertility specialist. The recipient undergoes a thorough evaluation to assess the health of her uterus, including blood tests to evaluate hormone levels and an ultrasound to examine the uterus and ovaries. A psychological assessment may also be recommended to ensure that the recipient is emotionally prepared for the use of donor eggs. Additionally, the donor undergoes her own medical screening, which typically includes genetic testing, infectious disease screening, and a fertility assessment to ensure that her eggs are of high quality.
Once the recipient’s medical evaluation is complete, the next step is selecting an egg donor. Donors are typically chosen from a donor bank, where their profiles, including medical history, genetic background, and physical traits, are available. Some recipients may choose to use an anonymous donor, while others may opt for a known donor (depending on the country’s law). The choice of donor is important, as it impacts both the genetic makeup of the embryos and the recipient’s emotional connection to the process.
The egg donor undergoes a process of ovarian stimulation, where she takes hormone injections (typically using gonadotropins) to encourage her ovaries to produce multiple eggs rather than the single egg that would be released in a natural cycle. The donor’s progress is closely monitored with blood tests and ultrasounds to track follicle growth. Once the follicles reach an optimal size (usually after 10-14 days of stimulation), a final injection of human chorionic gonadotropin (hCG) is given to trigger ovulation and the release of mature eggs.
Approximately 36 hours after the hCG injection, the donor undergoes an egg retrieval procedure, also known as oocyte aspiration. Under sedation, a needle is inserted through the vaginal wall and into the ovaries to collect the mature eggs. This is a minimally invasive procedure, and the donor is monitored during the recovery phase. Typically, multiple eggs are retrieved to increase the chances of successful fertilization.
Meanwhile, the recipient prepares for embryo implantation by undergoing hormone therapy, which typically includes estrogen and progesterone to stimulate the growth of the uterine lining (endometrium) and create an optimal environment for embryo implantation. This process may last several weeks, with regular monitoring to ensure the endometrium reaches the appropriate thickness for embryo transfer.
Once the eggs are retrieved from the donor, they are fertilized in the laboratory using sperm from the recipient’s partner (or a sperm donor, if necessary). The sperm and eggs are combined through conventional insemination or intracytoplasmic sperm injection (ICSI), where a single sperm is directly injected into each egg. The fertilized eggs (embryos) are cultured for several days, typically up to the blastocyst stage (5-6 days), to monitor their development and select the best quality embryos for transfer.
On the day of the embryo transfer, the best quality embryo is selected for implantation. A thin catheter is used to place the embryo into the recipient’s uterus, typically under ultrasound guidance to ensure precise placement. This procedure is usually painless, although some women may experience mild cramping afterward. If multiple embryos are available, the number transferred will depend on the age of the recipient and the quality of the embryos, as recommended by the fertility specialist.
After the embryo transfer, the recipient continues hormone therapy (e.g., progesterone) to support the uterine lining and facilitate implantation. About 10-14 days later, a blood test is conducted to check for pregnancy by measuring the levels of human chorionic gonadotropin (hCG), a hormone produced during pregnancy. If the test is positive, a follow-up ultrasound is typically scheduled after two weeks to confirm the pregnancy and check for a heartbeat. If the test is negative, the recipient may be advised to try another cycle.
If the pregnancy test is positive, the recipient will continue hormone treatment to maintain the pregnancy. The clinic will monitor the pregnancy with ultrasounds and blood tests to confirm its progression and detect any potential complications. Once the pregnancy is stable, care is typically transferred to an obstetrician for the remainder of the pregnancy.
IVF with egg donation is an effective treatment option for women who cannot use their own eggs. The process involves several stages, from selecting a donor and stimulating her ovaries to egg retrieval, fertilization, and embryo transfer into the recipient’s uterus. While egg donation IVF may require more steps than conventional IVF, it offers an opportunity for women who are struggling with infertility or age-related fertility decline to achieve a successful pregnancy using healthy donor eggs. With proper medical guidance and care, egg donation can provide a pathway to parenthood for many couples


Ovulation induction
Ovarian stimulation with timed intercourse (also known as “stimulated cycles with targeted intercourse”) is a fertility treatment designed to enhance the chances of conception by inducing the ovaries to produce multiple eggs, followed by well-timed intercourse. This method is often used for couples who have difficulty conceiving naturally. The process typically unfolds in several key stages:
The first step is a comprehensive evaluation of both partners’ fertility. This may involve blood tests to measure hormone levels, an ultrasound to assess the health of the ovaries and uterus, and a semen analysis to evaluate sperm quality. Based on these results, a treatment plan is developed.
To increase the number of eggs released during a woman’s cycle, ovulation is stimulated using fertility medications. The most common drugs used for this purpose are Clomiphene Citrate or injectable gonadotropins (e.g., FSH, LH). These medications encourage the ovaries to produce multiple follicles, each containing an egg, rather than just one egg as would occur in a natural cycle.
Throughout the stimulation phase, the woman undergoes regular monitoring through blood tests and ultrasounds. Blood tests track hormone levels (such as estradiol) to assess the ovaries’ response to the medications. Ultrasounds monitor the growth of the follicles to determine when they are ready for ovulation. Typically, the goal is to have 2-3 mature follicles for optimal chances of conception.
Once the follicles reach the appropriate size (usually around 18-20 mm), an injection of human chorionic gonadotropin (hCG) is administered to trigger ovulation. This hormone mimics the natural surge that would occur in a normal cycle, signaling the eggs to mature and be released from the follicles.
Intercourse are recommended on the same day of hCG injection and the day after. Timing intercourse at these moments maximizes the chances of sperm meeting the egg when it is released from the ovary.
About two weeks after ovulation, a blood test is performed to check for pregnancy. If the test is positive, a follow-up ultrasound is typically done around 6-7 weeks to confirm the pregnancy and assess its progress. If the result is negative, the couple can discuss next steps with their fertility specialist, which may include trying another cycle or considering alternative treatments.
Ovarian stimulation with timed intercourse offers a less invasive approach than more advanced fertility treatments like intrauterine insemination (IUI) or in vitro fertilization (IVF). The success rates depend on factors such as age, the underlying cause of infertility, and how the body responds to the stimulation. However, this method provides a more targeted way to enhance natural conception for many couples struggling with infertility.
Sperm donor
Assisted reproductive technology (ART) encompasses various treatments designed to help individuals and couples conceive a child when they are unable to do so naturally. When a male partner’s sperm is not available or viable, donor sperm can be used for treatments such as Intrauterine Insemination (IUI) and In Vitro Fertilization (IVF). These procedures allow individuals or couples to have a child using sperm from a donor, which is typically obtained from a sperm bank. Below is an overview of how IUI and IVF work when donor sperm is used.
Intrauterine Insemination (IUI) with Donor Sperm
Intrauterine insemination (IUI) with donor sperm is one of the simplest ART methods for achieving pregnancy. The process begins with the selection of a sperm donor. The donor sperm is obtained from a sperm bank, where it is rigorously screened for infectious diseases, genetic conditions, and quality. The sperm is then frozen and stored until it is needed for the IUI procedure.
- Ovulation Stimulation: In many cases, the woman undergoing IUI is given hormone treatment to stimulate her ovaries and produce one or more mature eggs. This is usually done with medications like Clomifene or injectable gonadotropins. The woman is closely monitored through ultrasounds and blood tests to track the development of the eggs and determine the best time for insemination.
- Sperm Preparation: On the day of the IUI procedure, the donor sperm is thawed and processed in the laboratory to separate the healthy, motile sperm from the semen. This increases the chances of fertilization and removes any unwanted components of the semen.
- Insemination: During IUI, a small amount of processed sperm is placed directly into the woman’s uterus using a thin, flexible catheter. This is done during her most fertile window, which is timed based on the monitoring of ovulation. The goal is to increase the sperm’s chances of reaching and fertilizing the egg by bypassing the cervix and placing the sperm closer to the fallopian tubes where fertilization typically occurs.
- Male Infertility: IUI with donor sperm is used when there is male infertility, including cases of azoospermia (no sperm) or severe sperm quality issues.
- Single Women or Same-Sex Couples: Single women or same-sex couples who wish to have a biological child may choose IUI with donor sperm as an option for conception.
- Medical Conditions: Conditions such as genetic disorders, chromosomal abnormalities, or other factors that make using a partner’s sperm unviable.
In Vitro Fertilization (IVF) with Donor Sperm
- Ovarian Stimulation and Egg Retrieval: The first step of IVF with donor sperm involves stimulating the woman’s ovaries to produce multiple eggs using hormone injections. Monitoring through ultrasounds and blood tests is done to track follicle development. Once the eggs are mature, they are retrieved through a minimally invasive procedure called oocyte pick-up, where a needle is used to collect the eggs from the ovaries.
- Fertilization with Donor Sperm: Once the eggs are retrieved, they are fertilized with donor sperm in the laboratory. In some cases, conventional insemination is used, where sperm is added to the eggs and fertilization is allowed to occur naturally. However, in some cases, intracytoplasmic sperm injection (ICSI) is often used. In ICSI, a single sperm is directly injected into an egg.
- Embryo Culture and Selection: The fertilized eggs are monitored for several days to allow the embryos to develop. The embryologist evaluates the embryos based on their quality and developmental stage. Typically, embryos are cultured to the blastocyst stage (5-6 days) to maximize the chances of implantation.
- Embryo Transfer: Once the embryos have developed, the best-quality embryo is selected for transfer into the woman’s uterus. This is done using a thin catheter and is a relatively simple and painless procedure. Depending on the circumstances, one or two embryos may be transferred, and any remaining healthy embryos can be frozen for future use.
- Post-Transfer Care and Pregnancy Test: After the embryo transfer, the woman continues hormone therapy (usually progesterone) to support the uterine lining and facilitate embryo implantation. A blood test is performed around 10-14 days after the transfer to check for pregnancy. If the test is positive, an ultrasound is scheduled to confirm the pregnancy and assess its viability.
- Severe Male Infertility: IVF with donor sperm is often the preferred option when male infertility is severe, such as in cases of azoospermia or very poor sperm quality that cannot be overcome with IUI.
- Single Women or Same-Sex Couples: Single women or same-sex couples may opt for IVF with donor sperm if they wish to have a child and prefer the IVF process over IUI.
- Medical Conditions or Genetic Concerns: Couples with genetic conditions, or when a woman is at risk of passing on a genetic disorder, may choose IVF with donor sperm to avoid transmitting these conditions. In some cases, genetic screening of the embryos can be done to ensure they are free of hereditary diseases before implantation.
- Woman indication for IVF: In all cases that IUI with donor sperm are indicated but in woman with IVF indication (tubal defect, severe endometriosis, pelvic abnormalities…)
Both IUI and IVF with donor sperm offer effective solutions for individuals or couples who require sperm from a donor to achieve pregnancy. IUI is less invasive and typically recommended for women with normal ovarian reserve and open fallopian tubes, while IVF is more complex and offers higher success rates, particularly in cases of severe male infertility or more complicated fertility issues. Donor sperm from sperm banks is rigorously screened for genetic and infectious diseases to ensure the safety and health of the child. Both treatments provide hope for single women, same-sex couples, and couples facing male infertility, offering the opportunity for biological parenthood.


Egg freezing
Egg freezing, also known as oocyte cryopreservation, is a process in which a woman’s eggs are harvested, frozen, and stored for future use. Increasingly, women are choosing to freeze their eggs for social reasons—such as focusing on their careers, pursuing education, or simply not yet finding the right partner—without the immediate pressure of fertility decline due to age. This procedure allows women to preserve their fertility and have the option of using their own eggs to conceive later in life. The egg freezing process generally consists of several key steps:
The first step in the egg freezing process is an initial consultation with a fertility specialist. During this meeting, the doctor will discuss the woman’s reasons for egg freezing, medical history, and any potential fertility concerns. The doctor will also perform an assessment that includes blood tests to measure hormone levels (e.g., AMH – Anti-Müllerian Hormone, which gives an estimate of ovarian reserve), as well as an ultrasound to examine the ovaries and assess the quantity and quality of the remaining eggs. This evaluation helps the doctor understand the woman’s fertility status and determine the best approach for her egg freezing cycle.
To maximize the number of eggs retrieved, the woman undergoes ovarian stimulation using hormone injections. The most commonly used medications are gonadotropins, which stimulate the ovaries to produce multiple eggs rather than the single egg that would be released in a natural cycle. These hormones typically include follicle-stimulating hormone (FSH) and luteinizing hormone (LH). The stimulation process lasts about 10-14 days and is carefully monitored through regular blood tests and ultrasounds to track how the ovaries are responding. The goal is to develop multiple mature eggs for retrieval.
During the ovarian stimulation phase, the fertility clinic closely monitors the woman’s response to the medication. Ultrasound scans are used to check the growth of the ovarian follicles (fluid-filled sacs that contain the eggs), while blood tests measure hormone levels like estrogen and progesterone to ensure optimal follicle development. Based on the results, the medication dosage may be adjusted to maximize egg production while minimizing the risk of ovarian hyperstimulation syndrome (OHSS), a potential side effect of the stimulation.
When the follicles have reached the appropriate size (usually around 18-20 mm), an injection of GnRH agonist and/or human chorionic gonadotropin (hCG) is administered to trigger ovulation. This step prepares the eggs for retrieval. Ovulation is typically triggered 36 hours before the planned egg retrieval, which ensures that the eggs are mature and ready for collection.
Approximately 36 hours after the ovulation triggering, the woman undergoes the egg retrieval procedure, known as oocyte pick-up. This procedure is performed under light sedation or anesthesia and is minimally invasive. A thin needle is inserted through the vaginal wall and into the ovaries to harvest (remove) the mature eggs from the follicles. The procedure usually takes about 20-30 minutes. The woman may experience some cramping or discomfort after the procedure, but recovery is generally quick.
After the eggs are retrieved, they are carefully examined and prepared for freezing. The eggs are then cryopreserved through a process called vitrification, which involves rapidly freezing the eggs to prevent ice crystal formation and preserve their integrity. The eggs are stored in a cryogenic freezer at sub-zero temperatures until the woman is ready to use them in the future.
After the egg retrieval, the woman may be monitored for a short time to ensure there are no complications, such as ovarian hyperstimulation syndrome (OHSS). She may be advised to rest for the remainder of the day. In some cases, the woman may need a follow-up visit to ensure that her cycle has returned to normal.
When the woman is ready to use her frozen eggs—whether in her 30s, 40s, or later—the eggs are thawed and fertilized with sperm (either from a partner or a donor) through in vitro fertilization (IVF). The resulting embryos are cultured and then transferred into the woman’s uterus, with the goal of achieving pregnancy. The number of eggs retrieved and successfully frozen will impact the likelihood of a successful pregnancy later, so multiple rounds of egg retrieval may be recommended, especially if the woman is in her late 30s or older.
Frozen eggs can be stored for many years, depending on the fertility clinic’s regulations and the woman’s preferences. Many clinics allow egg storage for up to 10 years, and some offer options to extend storage beyond this period, with regular updates and consent renewal required from the woman.
Egg freezing for social reasons provides women with the flexibility to delay childbearing without sacrificing their fertility potential. By freezing eggs at a younger age, women can preserve higher-quality eggs, increasing their chances of successful fertilization and pregnancy in the future. While the process requires careful planning, hormonal stimulation, and monitoring, it offers an empowering option for women who wish to balance their personal, professional, and family aspirations without the pressure of biological age constraints.
Recurrent Pregnancy Loss
Recurrent Pregnancy Loss (RPL) is typically defined as the occurrence of three or more consecutive pregnancy losses (miscarriages) before the 20th week of gestation. This condition is also referred to as recurrent miscarriage or habitual abortion. It affects approximately 1-2% of couples trying to conceive, although the exact cause can be difficult to pinpoint in many cases.
Causes of Recurrent Pregnancy Loss
- Chromosomal Abnormalities: One of the most common causes of RPL is chromosomal abnormalities in the embryos, often arising from either the egg or sperm. These can lead to issues like aneuploidy, where the embryo has too many or too few chromosomes, resulting in miscarriage.
- Parent Chromosomal Translocations: In some cases, one or both parents may carry a balanced translocation, where parts of chromosomes are rearranged but not missing. This can cause an unbalanced chromosomal distribution in the offspring, leading to miscarriage.
- Uterine Abnormalities: Structural problems in the uterus, such as a septate uterus (a partition in the uterus), fibroids, adhesions, or congenital malformations, can interfere with the implantation and growth of the embryo.
- Cervical Insufficiency: A weakened or incompetent cervix that opens prematurely can lead to a late miscarriage, particularly in the second trimester.
- Polycystic Ovary Syndrome (PCOS): Women with PCOS may have hormonal imbalances that affect ovulation, increasing the risk of miscarriage.
- Thyroid Disorders: Both hypothyroidism and hyperthyroidism can interfere with pregnancy maintenance, increasing the likelihood of RPL.
- Luteal Phase Defect: This condition which is not clearly defined, where the yellow body does not produce enough progesterone, can prevent the uterine lining from properly supporting the embryo.
- Antiphospholipid Syndrome (APS): An autoimmune disorder where the body produces antibodies that can lead to blood clotting, increasing the risk of pregnancy loss.
- Other Autoimmune Disorders: Conditions like lupus or rheumatoid arthritis can also increase the risk of miscarriage due to immune system abnormalities.
- Certain infections, such as bacterial vaginosis or other sexually transmitted infections (STIs), could possibly increase the risk of miscarriage by affecting the uterus and surrounding tissues.
- Smoking: Smoking is a well-established risk factor for miscarriage, as it can impair blood flow to the placenta and increase the risk of genetic abnormalities in the fetus.
- Excessive Alcohol or Drug Use: Both can negatively affect pregnancy outcomes, including increasing the risk of miscarriage.
- Obesity or Poor Nutrition: These factors can contribute to hormonal imbalances or other health problems that increase the likelihood of RPL.
- Inherited or Acquired Blood Clotting Disorders: Conditions like Factor V Leiden or protein C deficiency can increase the risk of blood clots forming in the placenta, which can disrupt blood flow and possibly lead to miscarriage.
As a woman ages, the quality of her eggs declines, which can lead to an increased risk of chromosomal abnormalities and miscarriage.
Both IUI and IVF with donor sperm offer effective solutions for individuals or couples who require sperm from a donor to achieve pregnancy. IUI is less invasive and typically recommended for women with normal ovarian reserve and open fallopian tubes, while IVF is more complex and offers higher success rates, particularly in cases of severe male infertility or more complicated fertility issues. Donor sperm from sperm banks is rigorously screened for genetic and infectious diseases to ensure the safety and health of the child. Both treatments provide hope for single women, same-sex couples, and couples facing male infertility, offering the opportunity for biological parenthood.